Statement - 1 : CH3 - CH2 - Cl + NaI Acetone→ CH3 - CH2 - I + NaCl Statement - 2 : Acetone is polar - protic solvent and solubility order
✓ Solved: When benzyl chloride is treated with sodium iodide in acetone, it reacts much faster than 1-chlorobutane,...
Thermodynamics Studies on the Solubility of Inorganic Salt in Organic Solvents: Application to KI in Organic Solvents and Water

Explain why 2-bromopropane react with sodium iodide in acetone over 10^4 times faster than bromocyclopropane. Hint: Examine the transition state for each of the reactions. | Homework.Study.com
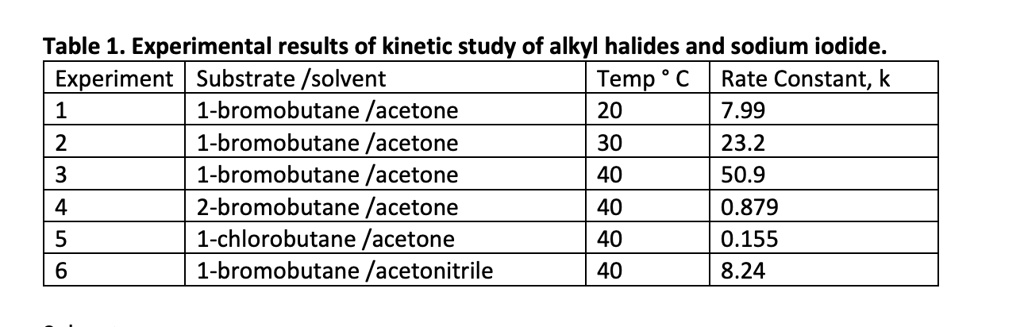
SOLVED: Table 1. Experimental results of kinetic study of alkyl halides and sodium iodide Experiment Substrate /solvent Temp C Rate Constant; 1-bromobutane / acetone 20 7.99 2 1-bromobutane /acetone 30 23.2 1-bromobutane /acetone
✓ Solved: When benzyl chloride is treated with sodium iodide in acetone, it reacts much faster than 1-chlorobutane,...
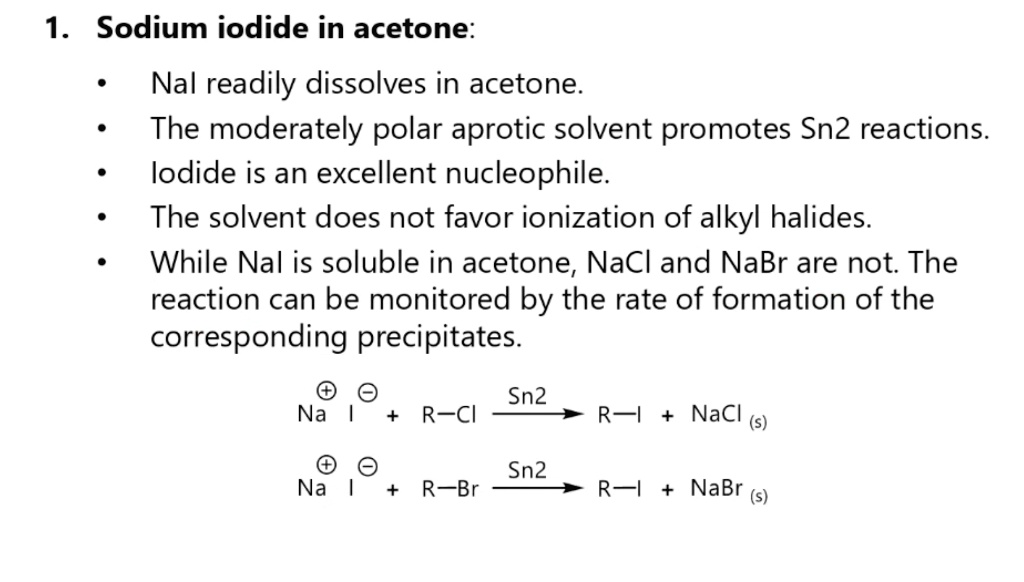
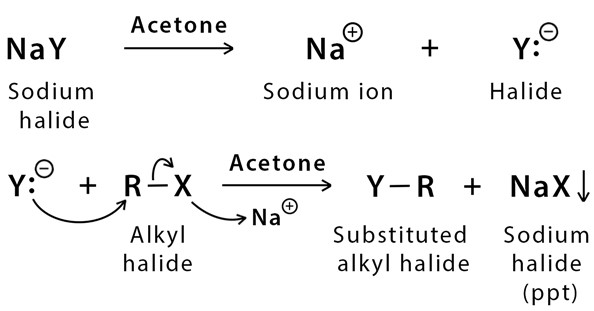
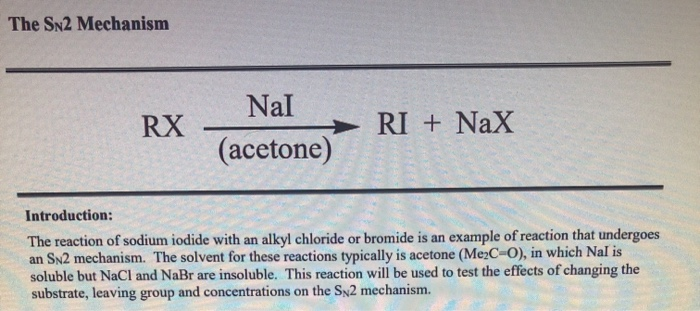
SOLVED: 1. Sodium iodide in acetone: Nal readily dissolves in acetone: The moderately polar aprotic solvent promotes Sn? reactions lodide is an excellent nucleophile: The solvent does not favor ionization of alkyl

SOLVED: In the first part of the experiment, you will react each substrate with sodium iodide in acetone. Acetone is a polar, aprotic solvent that promotes SN2 reactions. Iodide anion is a
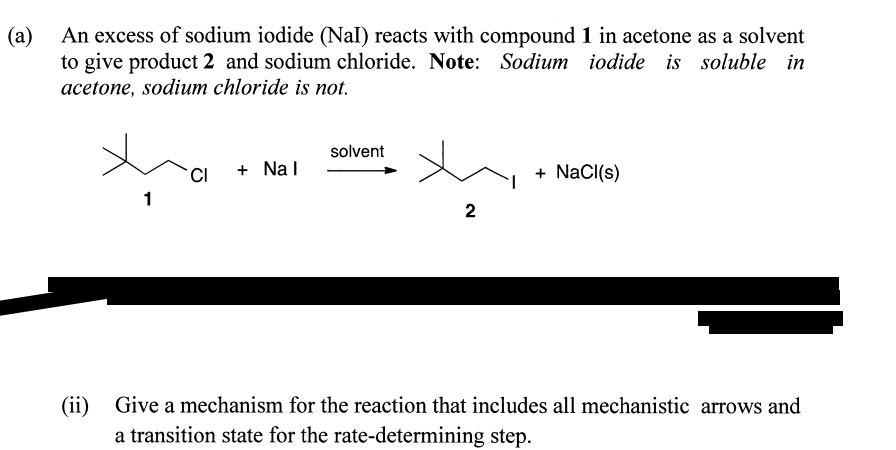
SOLVED: (a) An excess of sodium iodide (Nal) reacts with compound 1 in acetone as a solvent to give product 2 and sodium chloride. Note: Sodium iodide is soluble in acetone, sodium






![What is the name of the following reaction ? CH3CH2CH2Br [dry acetone]NaI CH3CH2CH2I What is the name of the following reaction ? CH3CH2CH2Br [dry acetone]NaI CH3CH2CH2I](https://i.ytimg.com/vi/hxgro16PgJg/maxresdefault.jpg)